Medical Device Process Validation. ISO 13485. IQ OQ PQ.
Why take this course?
🚀 Course Title: Medical Device Process Validation. ISO 13485. IQ OQ PQ.
🎓 Headline: Validate a Process to Gain Approval to Sell Medical Devices in the EU, U.S., & Internationally. Compliance to ISO 13485.
Unlock the Secrets of Medical Device Process Validation!
🎉 Course Overview: This comprehensive course is meticulously designed to demystify process validation for medical devices, guiding you through the intricacies of compliance with ISO 13485, FDA QSR, and the EU MDR. With six detailed sections, this course is an invaluable resource for anyone involved in the manufacturing or quality assurance of medical devices.
📚 Course Structure:
-
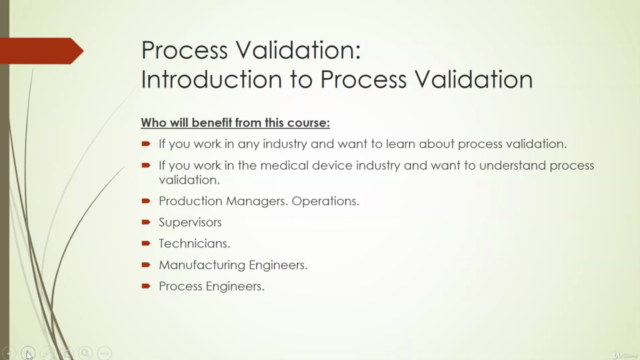
Introduction to Process Validation (Section 1)
- Understanding the necessity and importance of process validation.
- Determining the need for validation and its regulatory framework.
- A brief on the three types of validation: IQ (Installation Qualification), OQ (Operational Qualification), PQ (Performance Qualification).
-
The Nuts and Bolts of Process Validation (Section 2)
- Step-by-step guidance on performing process validation.
- Exploring the User Requirement Specification, Design Qualification, Install Qualification, Operational Qualification, and Performance Qualification.
- A live demonstration of an Operational Qualification.
-
Mastering Process Capability (Section 3)
- Grasping the concepts of process capability ratio, process capability index, and process model.
- Understanding control limits, action limits, and the role of Design of Experiments in Operational Qualification.
-
Risk Management (Section 4)
- Identifying the critical role of risk management within medical device regulation.
- Aligning risk management with the validation process and applying ISO 14971 principles.
-
Documentation for Process Validation (Section 5)
- A deep dive into essential documentation including:
- Process Validation Documentation.
- Validation Master Plan.
- Validation Plan.
- Validation Change Control.
- Calibration and Preventive Maintenance within the validation process.
- Understanding the Validation Summary Report.
- A deep dive into essential documentation including:
-
Regulatory Evolution in Life Sciences (Section 6)
- Tracing the development of U.S. regulation, understanding how process validation became a cornerstone in the life sciences industry.
🎯 Who Should Take This Course? This course is tailored for:
- Operations and People Managers
- Manufacturing, Process, and Development Engineers
- Quality Engineers
- Regulatory Affairs Professionals
- Research and Development Engineers
- Students seeking to understand process validation
- Entrepreneurs, SMEs, and Start-ups aiming to navigate the manufacturing process of medical devices.
📈 Why Enroll? This course stands out for its practical approach to a complex topic, breaking down process validation into clear, manageable sections. It's perfect for:
- Gaining a solid understanding of compliance with ISO 13485 and other international standards.
- Preparing for the challenges of selling medical devices in regulated markets.
- Building a strong foundation for process development and validation in any industry.
- Enhancing your career with specialized knowledge that is highly sought after by employers in the medical device sector.
📅 Start Your Journey Today! Embark on a transformative learning experience that will equip you with the knowledge and tools to successfully validate processes and navigate the complex regulatory landscape of medical devices. Enroll now and open the door to new opportunities and career advancement in the ever-evolving field of medical device manufacturing.
Course Gallery



Loading charts...
Comidoc Review
Our Verdict
This course offers extensive knowledge on process validation and related regulations, making it a valuable resource for professionals in medical device or pharmaceutical industries looking to improve their understanding. However, potential learners should be prepared for occasional quiz phrasing issues and recognize that certain topics might not receive the depth of treatment they expected.
What We Liked
- Comprehensive coverage of process validation, IQ, OQ, PQ, and industry-specific regulations like ISO 13485 andCode of Federal Regulation (21 CFR section 820)
- Well-structured course with clear objectives, presentation of topics, quiz items, and summary for each lesson
- Complements technical information with practical examples, making complex concepts more accessible
- Includes a wide array of resources, such as the Validation Master Plan and Design of Experiments
- Caters to beginners and professionals alike from life science industries, including medical devices and pharmaceuticals
Potential Drawbacks
- Quiz questions have been reported to be inconsistent in their phrasing and may cause confusion for some learners
- Lacks depth in certain areas like real-world examples, risk management, process capability indices (CP & CPK), and test method validation topics
- ISO 13485:2016 standard is not referenced directly, as the course relies on an older version for specific clauses
- Presentation style has been described as monotonous by a few learners