Pharmaceutical Clinical Bioequivalence Study (BA/BE) Course

Why take this course?
🚑 Master the Art of Clinical Bioequivalence Studies with Our Expert-Led Course!
Understanding the Significance of Bioequivalence Studies in Pharmaceuticals 🚀
Bioequivalence studies (BA/BE) are a cornerstone in the pharmaceutical industry, ensuring that generic medications deliver the same efficacy and safety as their brand-name counterparts. These studies are crucial for monitoring the pharmacokinetic (PK) and pharmacodynamic (PD) parameters of drugs post-administration, providing critical data for regulatory submissions and drug approval processes.
Dive into Bioavailability & Bioequivalence 💧
-
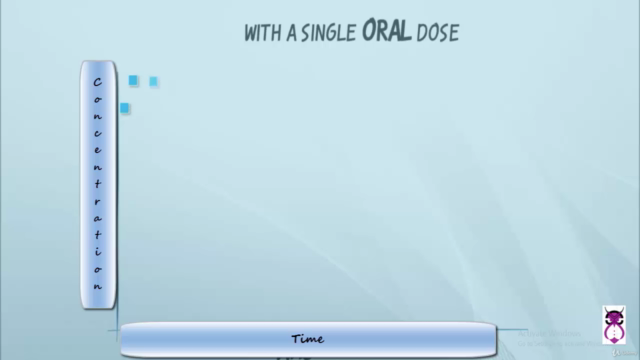
Bioavailability (BA) is the extent (quantitative aspect) and rate (qualitative aspect) at which the active substance from a pharmaceutical product becomes available to the systemic circulation. Understanding bioavailability is vital in drug development, as it affects how the body will respond to the medication.
-
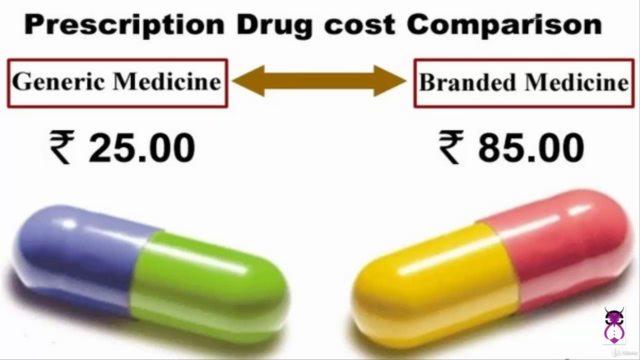
Bioequivalence (BE) demonstrates that two medications contain the same amount of medicine and work the same way in the body as their brand-name equivalent. This is critical when submitting a generic dossier, ensuring that generics are not only bioavailable but also bioequivalent to the original drug.
Course Overview & Outcomes 📚
This comprehensive course by Mahesh Pratap will guide you through every nuance of BA/BE studies, from the regulatory landscape to the practicalities of design, data analysis, and reporting. By the end of this course, you'll have a deep understanding of bioavailability, enabling you to confidently design and execute your own bioequivalence studies for generic drug approvals.
What You Will Learn 🎓
-
Designing Studies: Understand how to create a new drug or new device study, with a focus on establishing statistical equivalence to an existing treatment.
-
Regulatory Compliance: Learn how to design a study that meets the stringent regulatory requirements of the pharmaceutical industry.
-
Data Analysis Techniques: Gain proficiency in analyzing pharmacokinetic (PK) data and conducting dose-response analysis, fitting statistical models for average patient response.
-
Bioequivalence Designs: Master both parallel and crossover designs, understanding their strengths and applications.
-
Real-World Application: Review actual clinical trials to identify endpoints, questions of interest, and the statistical methods employed.
Course Structure & Content 📐
-
Basic Concepts for Bioequivalence Study
-
Bioavailability & Bioequivalence
-
Types of Study Designs
-
Generic Medicine Equivalency with Branded Medicines
-
Criteria and Considerations for BA/BE Study
-
Pharmacokinetic (PK) Analysis
-
Dose-Response Analysis
-
Case Studies Review
Who Can Benefit from This Course? 👩🏫👨💼
This course is designed for:
-
Diploma Pharmacy Students/Professors: Enhance your teaching and research skills in bioequivalence studies.
-
Pharmacy Students: Gain a solid foundation in clinical pharmacokinetics and biostatistics.
-
Pharmaceutical Researchers & Investors: Understand the financial implications of successful BA/BE studies.
-
Doctors: Stay informed on the latest clinical trial methodologies for better patient outcomes.
-
Medical Professionals: Get a grasp on how to interpret and apply data from bioequivalence studies.
-
Analysts: Acquire the tools to design, implement, or analyze clinical trials with confidence.
Embark on Your Journey to Expertise in Bioequivalence Studies Today! 🌟
Join us and transform your understanding of pharmaceutical development. With this course, you'll be equipped with the knowledge and skills to navigate the complexities of bioavailability and bioequivalence, ensuring you stay at the forefront of pharmaceutical science. Enroll now and take the first step towards becoming a Bioequivalence Study expert! 🎉
Note: This course is designed for learners who have a foundational understanding of pharmacokinetics and pharmacodynamics, as well as some knowledge of statistical methods in clinical trials.
Course Gallery


Loading charts...