Good Clinical Practice ICH GCP - Full Certificate Course
Good Clinical Practice, ICH-GCP, E6 (R2+R3), Good Clinical Practice, IRB/IEC, Investigator, Sponsor
4.15 (236 reviews)

529
students
2.5 hours
content
Nov 2024
last update
$49.99
regular price
What you will learn
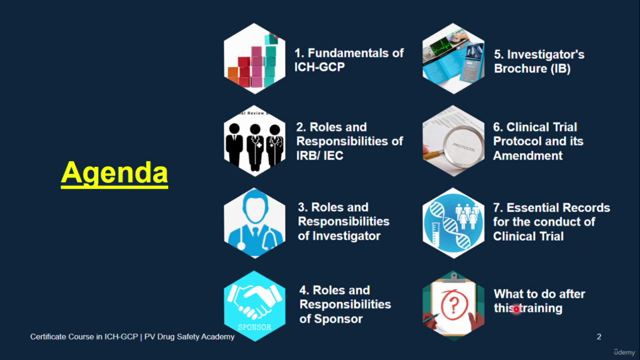
Fundamentals of ICH-GCP
Principles of ICH-GCP
Roles & Responsibilities of IRB/IEC
Roles & Responsibilities of Investigator
Roles & Responsibilities of Sponsor
Investigator's Brochure (IB)
Clinical Trial Protocol and its Amendment
Essential Records for the conduct of Clinical Trial
Course Gallery

Loading charts...
5675576
udemy ID
24/11/2023
course created date
14/07/2024
course indexed date
Bot
course submited by